MedTech Startup Compliance : Your Guide to FDA & HIPAA Regulations
The medical technology sector is a promising but challenging industry for startups. With global MedTech revenues projected to reach $694.7 billion by 2025, the opportunities are immense. However, MedTech startup compliance is a key challenge. It can either help or break new companies trying to launch innovative healthcare solutions.
Understanding the Regulations: FDA and HIPAA
Before we get into the details, we should first understand the roles of these two MedTech regulatory requirements.
The FDA is accountable for safeguarding public health through the regulation of the safety, security, and efficacy of human and animal drugs, biologicals, and medical devices. HIPAA is a United States federal statute to protect personal patient health data from release without the knowledge or agreement of the patient. With MedTech devices still gathering, storing, and transmitting health information, compliance with HIPAA cannot be avoided.
FDA & HIPAA compliance solutions for startups may be necessary as most modern medical devices deal with electronic Protected Health Information (ePHI). Familiarity with these MedTech regulatory demands from the very beginning is the key to establishing a sustainable and reputable business.
Demystifying FDA Compliance for Medical Devices
The FDA’s regulatory pathway for your device is determined almost entirely by its risk level. The agency uses a three-tiered classification system for FDA compliance for medical devices.
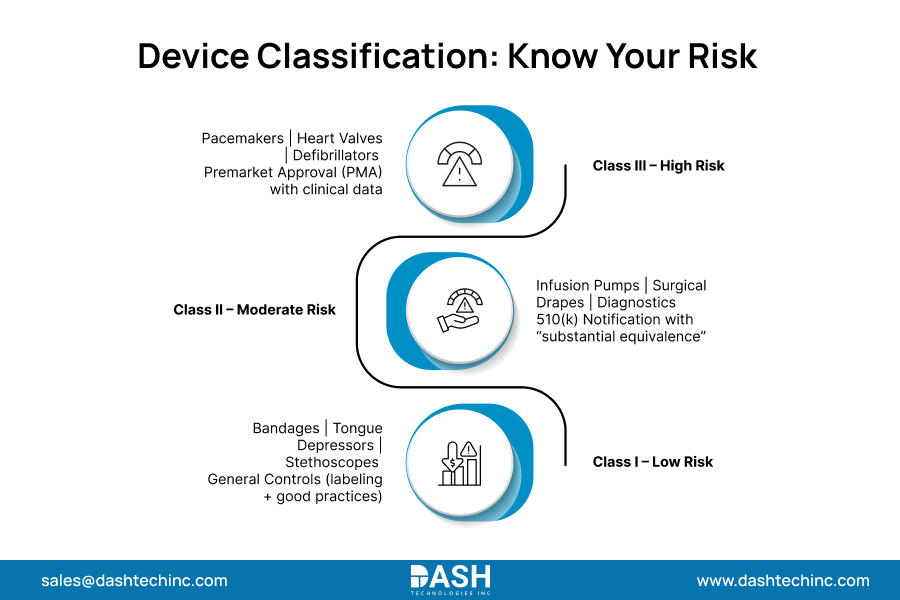
Class I (Low Risk): These devices are less likely to pose any danger to the user. Examples include elastic bandages, tongue depressors, and manual stethoscopes. Most Class I devices don’t need premarket submissions. However, they must follow general controls like proper labeling and good manufacturing practices.
Class II (Moderate Risk): This is the largest device category. Most likely to use a 510(k) Premarket notification. This requires “substantial equivalence” to already legally marketed devices—examples: infusion pumps, surgical drapes, most diagnostic instruments.
Class III (High Risk): These devices have the highest risk and need to go through the premarket approval (PMA) process. Premarket approval devices must be supported by clinical testing and complete analyses of their safety and effectiveness before they are marketed for use. These are usually life-supporting or life-sustaining devices. Pacemakers, heart valves, and automated external defibrillators (AEDs) are a few examples.
Compliance Made Simple for MedTech Startups
FDA & HIPAA regulations can be complex, but the right guidance helps your MedTech startup move faster, safer, and smarter.
Start Your Compliance Journey
Regulatory Pathways
Understanding your device’s class is crucial because it dictates your path to market:
- 510(k) Premarket Notification: The most common route for MedTech startups. To achieve approval for a 510(k), you’ve got to demonstrate your new device is “substantially equivalent” (i.e., just as safe and effective) to an older model of a device. That older one? A “predicate device.” It doesn’t need to be an exact duplicate, but it does need to perform the same function & possess similar tech specifications.
- Premarket Approval (PMA): The “gold standard” of FDA review. Only for Class III devices. A PMA application is based on a significant amount of scientific evidence, including data from clinical trials, to ensure the device is safe and effective. This is a long and expensive process.
- De Novo Classification Request: What if your device is novel and low-to-moderate risk, but there’s no predicate device to compare it to for a 510(k)? The De Novo pathway is for you. It allows the FDA to classify a novel device as Class I or II, creating a new regulatory category for future devices of its kind.
HIPAA Compliance for MedTech Startups
Understanding HIPAA’s Scope for Startups
HIPAA compliance for MedTech startup is needed for all entities that store, collect, transmit, or process Protected Health Information (PHI). Most MedTech startups are considered a “Business Associate” and perform work on behalf of covered entities like hospitals, clinics, or healthcare providers.
MedTech compliance consulting services are required by startups creating telehealth services, digital health platforms, medical devices, and AI-powered healthcare technologies, as they are subject to the Health Insurance Portability and Accountability Act. The ecosystem of partners and subcontractors who might have access to PHI is covered by this MedTech startup compliance, which goes beyond simply managing patient data.
Core HIPAA MedTech Regulatory Requirements
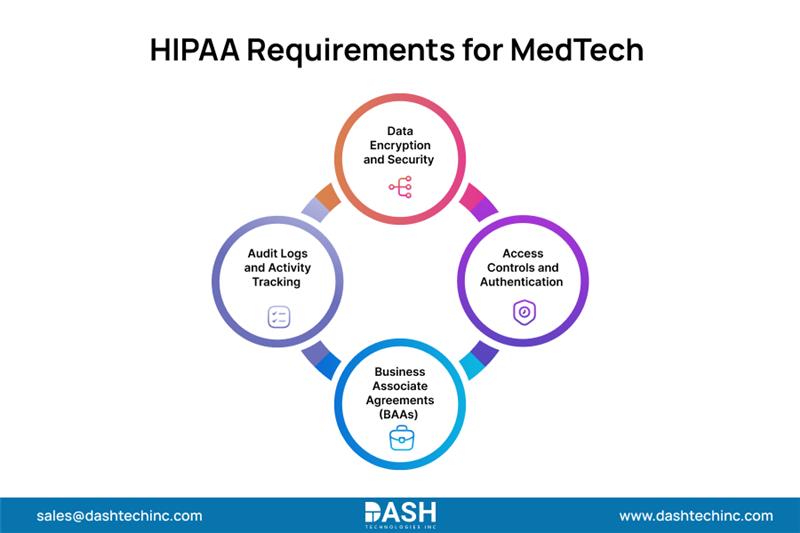
Data Encryption and Security: All PHI needs to be encrypted using industry standards such as TLS 1.2+ for data transmission and AES-256 for stored data. To ensure complete protection throughout the data lifecycle, this HIPAA compliance for MedTech startup requirement applies to data both at rest and in transit.
Access Controls and Authentication: Restricting access to PHI to authorized personnel using multi-factor authentication (MFA), role-based access control (RBAC), and automatic logoff features; these safeguards keep track of who accessed what data when & help prevent unwanted access.
Business Associate Agreements (BAAs): Startups must enter into proper BAAs with any cloud services that handle PHI, for example, cloud hosting services, AI model services, and APIs. They laid out acceptable uses of PHI and liability models for breach scenarios.
Audit Logs and Activity Tracking: HIPAA mandates the creation of detailed, unalterable records of PHI access that are securely maintained for a minimum of six years. Beyond evidence of MedTech startup compliance, the records can be utilized for tracking probable security breaches.
The Cost of Non-Compliance
HIPAA violations can lead to fines of up to $1.5 million for each violation. There may also be criminal charges and a loss of credibility. For startups with tight budgets, these penalties can be severe. Non-compliance can also cause loss of investor confidence. It can cause problems when working with healthcare organizations and exclusion from major market opportunities.
Developing a Strategic Compliance Approach
Accelerating MedTech innovation demands designing regulatory compliance into products from day one. “Security by design” is a key FDA priority, requiring early cybersecurity & privacy safeguards instead of retroactive patches. That reduces long-term costs and ensures medical devices stay aligned with emerging MedTech regulatory requirements.
HIPAA Compliance for MedTech Startup Success
Startups should design systems with encryption, access controls, and audit logging from the start to ensure HIPAA compliance for MedTech startup operations.
Risk-Based Strategy
- Conduct risk assessments to identify vulnerabilities.
- Tailor mitigation strategies to the product scope and market.
- Correctly classify devices to streamline FDA compliance for medical devices.
Cost-Effective Compliance Options
- Partner with MedTech compliance consulting services.
- Use consultants instead of costly full-time officers.
- Leverage tools for FDA & HIPAA compliance solutions for startups.
This ensures a level of compliance without compromising too much on innovation and with more manageable compliance costs.
The Path Forward for MedTech Startups
Although MedTech startup compliance may seem like an uphill battle at times, viewing compliance as an advantage to be leveraged rather than a necessary evil to be endured can be an excellent motivator; for building better and faster, your commitment to compliance will not only gain the trust of your patients, providers, and investors, but it can also be a major differentiator in a crowded marketplace.
MedTech startups can create better devices by using compliance-by-design thinking. This approach helps reduce risks and makes the path to market easier. The key is to do it early and stay ahead by being prudent and seeking expertise when necessary.
Startups dealing with these complexities can find MedTech compliance consulting services very helpful. Get in touch today! Our expert services can help you face challenges and make your vision a reality.
About Dash

Dash Technologies Inc.
We’re technology experts with a passion for bringing concepts to life. By leveraging a unique, consultative process and an agile development approach, we translate business challenges into technology solutions Get in touch.