Building Compliant and Secure AI for MedTech : HIPAA, ISO, and FDA Standards
The Hidden Price of Non-Compliance
Non-compliance in AI-enabled healthcare systems is never just a regulatory line item—it compounds into breach of risks, operational disruption, reputational damage, delayed clearances, and elevated cost of capital when investor confidence drops. HIPAA enforcement and breach trends underline a sustained increase in large hacking incidents and ransomware, which directly translate into service interruptions and emergency responses that drain both clinical and engineering resources.
Attackers recently hit scheduling platforms, diagnostic feeds, and patient databases while companies raced to close security holes under regulatory watch. The costs multiply fast. Fines and legal bills are just the start. Engineering teams abandon feature work to fix old issues. Launch dates slip. New AI features sit unused while teams’ complete validation and add controls.
The penalty problem: Violations bring hefty fines of up to $1.5 million and force corrective plans onto teams already handling complex timelines and documentation.
The clearance problem: Finding compliance gaps during audits or reviews means rebuilding quality procedures, risk files, security designs, and governance rules. Each rebuild pushes back approvals.
The confidence problem: Security incidents or murky AI/ML regulatory plans signal risk, which can slow funding or partnerships when timing matters.
Build Your Compliant AI Solution
Get Dash’s proven compliance-first framework to align your AI product with HIPAA, ISO, and FDA standards—before costly rework begins.
Book a Free ConsultationThree Regulatory Standards: HIPAA, ISO 13485, and FDA
Regulatory expectations for AI in MedTech align around three pillars. First: protecting patient data through strong privacy and security. Second: running tight quality systems for medical device software. Third: Proving AI/ML systems stay safe and effective in real use. Meeting these three creates overlapping needs for governance, documentation, validation, risk management, post-launch monitoring, and change controls. Smart companies incorporate these into AI development from day one, rather than adding them later.
HIPAA Security Rule
This law requires three layers of safeguards for electronic protected health information (ePHI): administrative, physical, and technical. Your organization must perform risk assessments. Access controls limit who sees patient data. Audit logs track every access event. Data in transit must be encrypted. And contingency plans address what happens when systems fail.
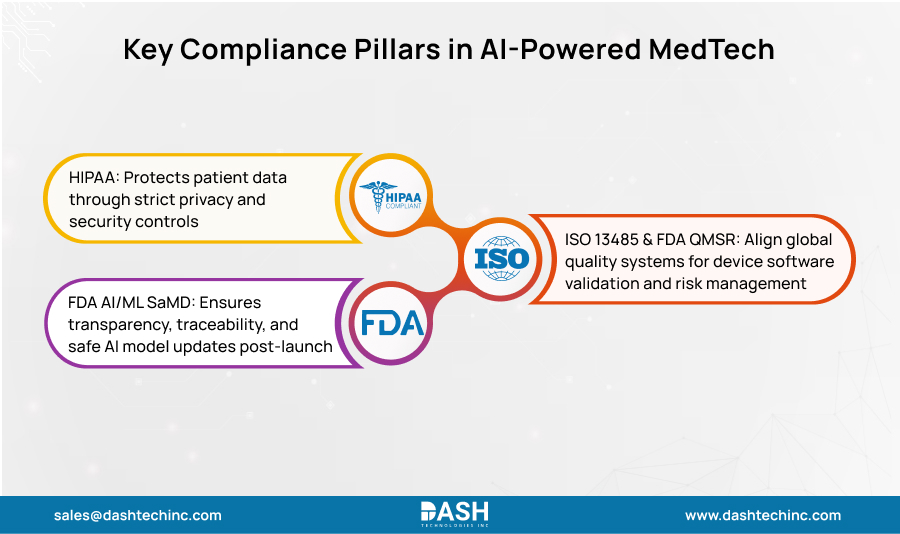
ISO 13485 and FDA QMSR
FDA’s 2024 Quality Management System Regulation (QMSR) brought U.S. device quality standards in line with ISO 13485:2016. What has changed? Better consistency across design controls, risk management procedures, validation requirements, and production oversight for device software- Software as a Medical Device (SaMD) included. Organizations seeking FDA AI approval now work within a more predictable framework.
FDA AI/ML SaMD
FDA’s guidance on AI in Software as a Medical Device emphasizes key priorities. Transparency: regulators need to see your development process. Traceability: decisions and data must be documented. Validation testing should match risk levels. Risk management extends past approval through the full product lifecycle. The Predetermined Change Control Plan (PCCP) stands out—it lets you update AI systems that learn continuously after launch while maintaining HIPAA AI compliance and meeting AI regulatory compliance in MedTech standards.
Dash Technologies: Building Compliance in from the Start
Dash Technologies does not bolt compliance at the end. It’s designed from the ground up. Dash’s Compliance-First AI Development Framework embeds HIPAA, ISO 13485, and FDA standards throughout the entire pipeline, from ideation through to production.
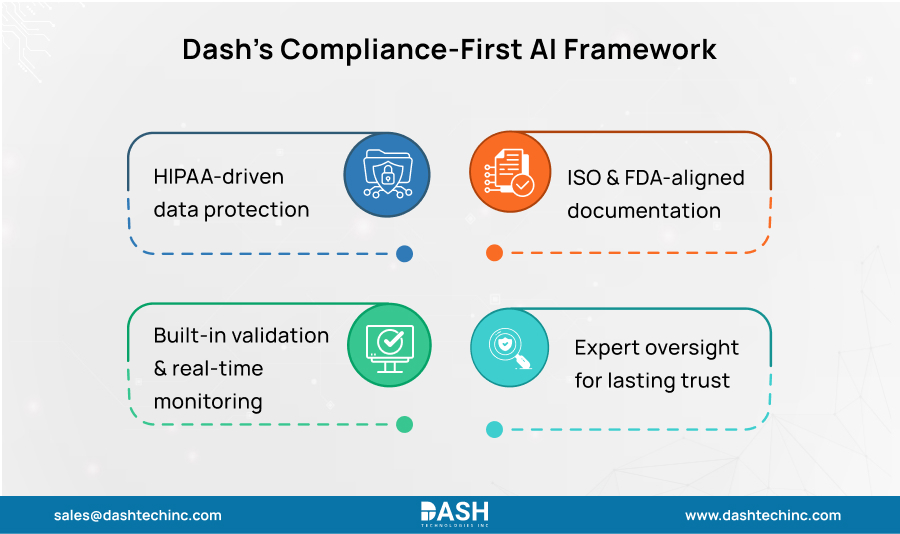
Five core pieces make up the framework:
1. Protected Data Infrastructure
Dash starts with HIPAA requirements when building data systems. Role-based access controls decide who sees what data. Encryption guards’ data whether stored or moving. De-identification strips identifying info where needed. Each step from collection to predictions includes barriers against unauthorized access and keeps full audit records.
2. Ready-for-Review Documentation
Every AI component gets documentation that meets ISO 13485 and FDA standards. This covers data prep, model design choices, training steps, and output interpretation. Full traceability makes regulatory inspections and submissions smoother.
3. Built-In Validation
Dash adds validation checkpoints throughout model development to hit FDA marks for explainability, reproducibility, and strength. Core pieces include:
- Version control for datasets and models
- Automated validation reports
- Tools to catch bias and performance drift
4. Continuous Monitoring After Launch
Live systems get ongoing monitoring for performance drops, security holes, and regulation changes. Dash’s DevOps and QA teams keep compliance current as systems and rules evolve.
5. Expert Team Partnerships
Dash collaborates with regulatory consultants, healthcare compliance specialists, and legal counsel to ensure deliverables are in line with current compliance and future readiness of FDA, HIPAA, and ISO standards.
Case Examples: From Prototype to FDA-Ready AI Device
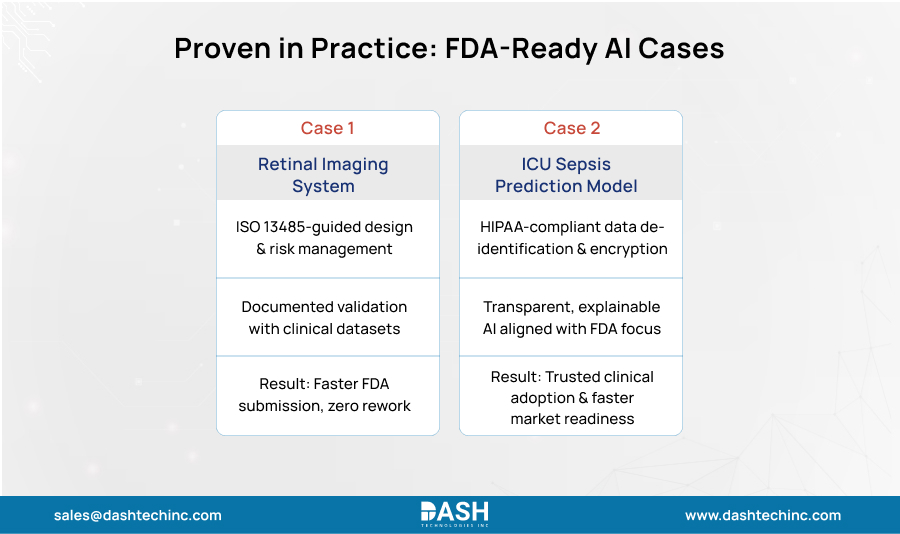
The true value of a compliance-first approach is best illustrated through real-world applications. Here are two examples of how integrating compliance early leads to better outcomes.
Case One: Retinal Imaging Diagnostic System
A device startup built an algorithm to spot early signs of a specific eye condition from retinal scans. Using ISO 13485 principles from the start of the project gave them a proper development structure.
Design controls: Development went through formal stages. The team turned the ophthalmologist’s needs into technical specs. Design reviews happened before any coding.
Risk work: Systematic risk review tackled key questions. What is the risk of a false negative? How does it handle poor image quality? The team has mitigation plans into software design itself. This proactive AI risk management was crucial for demonstrating device safety.
Validation: Testing used preset datasets with performance targets set by clinical advisors. All testing protocols and results were meticulously documented, creating a clear and defensible validation file for regulators.
Result: A smooth and successful submission process, as their documentation already met the stringent requirements for medical device software compliance.
Case Two: ICU Sepsis Prediction System
A healthcare group built a predictive model to flag ICU patients at high sepsis risk using electronic health record data.
HIPAA Compliance: Step one was the creation of a de-identified training dataset, removing all 18 personal identifiers in compliance with the HIPAA Safe Harbor method. The live application was designed with a secure, HIPAA-compliant cloud platform so that patient information was encrypted and only accessible to permitted clinicians.
FDA transparency: Knowing clinicians need to trust AI guidance; they are built for openness. Instead of just flagging high-risk patients, the system shows contributing factors like elevated lactate levels or rising white cell counts. This matches the FDA’s focus on explainable AI. The team constantly measures model performance against accepted clinical benchmarks.
Both cases prove measurable gains and significant ROI benefits from early compliance focus: lower late-stage failure risk, smaller rework costs, and faster paths to market readiness and regulatory approval.
Ready to Build Your Compliant AI Solution?
Developing AI for Healthcare has immense regulatory challenges. The best companies are not simply stressing about this later, but are rather building HIPAA, ISO 13485, & FDA compliance into their plans from the start. This fundamental approach saves money while also accelerating market launch.
The alternative creates predictable trouble. Projects that postpone compliance work inevitably see their timelines stretch far beyond original estimates. Costs balloon beyond budget. In worst-case scenarios, entire initiatives collapse under regulatory burdens teams didn’t anticipate. Actual project data shows that a different path of work building compliance into early planning reduces time to market and improves financial returns.
We offer a Compliance Readiness Assessment to address these challenges. Our specialists examine your project’s current position, identify where regulatory requirements aren’t met, and provide specific steps forward. The assessment examines essential areas: strategies for AI risk management in healthcare environments, requirements for medical device software compliance, methods for implementing AI validation and testing standards correctly, and protections needed for healthcare AI data privacy.
Get your AI solution FDA ready with Dash’s compliance-first framework Schedule your assessment today.
About Dash

Dash Technologies Inc.
We’re technology experts with a passion for bringing concepts to life. By leveraging a unique, consultative process and an agile development approach, we translate business challenges into technology solutions Get in touch.