Predictive Analytics in Clinical Trials : Data-Driven Decisions
The pharmaceutical and biotech sectors remain beset by unprecedented challenges in getting innovative treatments to market. With trial expenses running a projected $1.3 billion per approved drug and failure rates of more than 90%, there has never been a greater imperative to make wiser, data-informed choices. Enter predictive analytics in clinical trials, a revolutionary method that applies artificial intelligence to change the face of research study design, execution, and analysis.
The Traditional Clinical Trial: Mess of Inefficiency
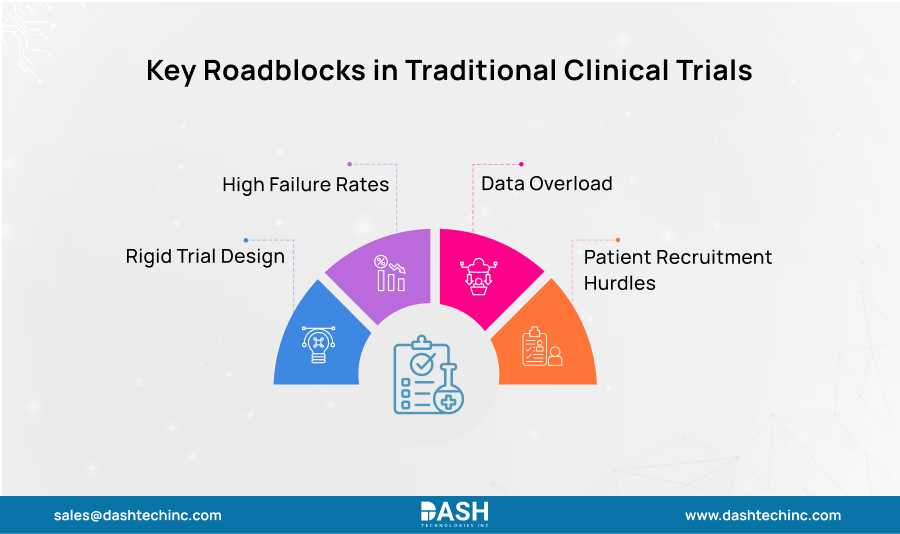
To appreciate the scale of the AI-driven revolution, it’s important first to understand the limitations of the conventional clinical trial model. For years, the process has been notoriously inefficient. A single drug can take years & billions of $ to bring to market, with a significant portion of that time & expense consumed by clinical trials. Historical outcomes, incomplete data sets, and even educated guesswork inform many of its growing pains.
Key roadblocks in the traditional process include:
- Suboptimal Trial Design: Trial protocols were sometimes inflexibly designed from the outset as a ‘one-size-fits-all’ approach. Protocols that are too rigid may waste time and money if interim results recommend a different approach.
- Patient Recruitment Hurdles: Recruitment & identifying the right patients have long been one of the biggest bottlenecks in drug development. Poor patient selection not only slows down trials but is a leading contributor to their failure. Researchers struggled to find homogeneous patient groups who were most likely to respond to a specific therapy.
- High Failure Rates: As mentioned above; most drugs that enter clinical trials fail to reach the market. The failures can occur at any phase of a trial, often due to unforeseen safety issues or a great lack of efficacy that could’ve easily been predicted with the help of clinical trial data analytics solutions.
- Data Overload: From genomic sequences & electronic health records (EHRs) to imaging & real-world evidence (RWE), modern trials produce a vast amount of complex data. It is nearly impossible to manually analyze this data in order to make timely decisions.
The consequences of these inefficiencies are not only increased costs but also delayed access to life-saving treatments for patients.
Drive Smarter Decisions with AI
From adaptive designs to early risk detection, see how predictive analytics can improve efficiency, accuracy, and trial success rates.
Let’s TalkWhat is Predictive Analytics?
Predictive analytics is the transition from hindsight to foresight. It uses statistical algorithms, machine learning (ML), and historical data to predict future outcomes. In clinical research, it extracts patterns from complex and heterogeneous datasets to drive insights, make predictions, and allow organizations to take action. AI in clinical research transforms raw data into a strategic asset.
The leap from raw data to actionable recommendations can be understood with a continuum of analytical skills:
- Descriptive Analytics (What happened?): This is the base layer, involving dashboards and reports that summarize retrospective data, for example, patient recruitment rate or adverse events of a previous quarter. It provides a meaningful representation of things that happened in the past.
- Diagnostic Analytics (Why did it happen?): The next level of analytics begins to uncover the root causes of what is happening in the data. For instance, if a trial site is underperforming with respect to enrollment, diagnostic analytics might reveal the reason, such as the inclusion criteria are too restrictive or that the demographics of the local patient population don’t match the target population for the trial.
- Predictive Analytics (What is likely to happen?): This is one of those inflection points wherein AI in decision-making begins to reach its full potential. Using machine learning algorithms trained on deep historical datasets, AI-augmented clinical studies software can predict future events with tremendous accuracy. Predictive analytics in clinical trials can detect patients at high risk of discontinuation, estimate times of enrollment, or calculate the probabilities of a drug intervention meeting its primary endpoints.
- Prescriptive Analytics (What should we do about it?): Most developed stage, prescriptive analytics, extends beyond prediction to suggest definitive action. In instances where a model forecasts a high likelihood of a specified adverse event, it may suggest different dosages or more frequent monitoring for a specified subgroup of patients. This is the pinnacle of data-informed decision-making.
With each stage of the continuum, clinical organizations can develop a strong clinical trial data analytics solutions platform that helps predict & hyper-optimize every aspect of the trial.
AI-Powered Predictive Analytics: Key Benefits
Optimizing Trial Design & Protocols
A successful trial begins with a robust and feasible protocol. AI is instrumental in getting this right from the very start.
- Predictive Feasibility: Clinical trial predictive modeling can virtually simulate trial performance even before the first patient is enrolled. Using predictive analytics in clinical trials, research teams can forecast the influence of protocol design on patient burden, site efficiency, and overall cost. This pre-planning capability enables the avoidance of expensive amendments and enrollment setbacks later.
- Adaptive Trial Designs: AI-driven clinical research software allows for more adaptive trial designs. An adaptive trial can be adjusted in response to interim data and analysis rather than following a strict protocol. For example, the trial can be modified to concentrate on that arm if a specific dosage is showing greater efficacy. This would make the study more ethical and efficient.
- Endpoint Selection: Endpoint selection is essential for demonstrating efficacy. AI in clinical research can help by quickly mining the historical data of thousands of previous trials to help researchers select endpoints most likely to be accepted by regulators & demonstrate a clinically meaningful benefit.
Smarter Patient Recruitment and Site Selection
Selecting the right patients and the right sites to run a trial is one of the foundational keys to success.
- Precision Recruitment: AI in decision-making can examine vast databases of information, such as electronic patient records, genomic data, and biomarkers, to identify patient cohorts best suited to a specific therapy. This would go beyond matching just basic demographics to a more targeted, individualized matching. In cancers, for example, it is already a routine technique, with patients being selected depending on specific genetic mutations.
- Identifying High-Performing Sites: Not all clinical sites perform the same. AI in clinical research can analyze historical site performance to identify high-performing sites with a track record of successful patient recruitment & data quality for a specific therapeutic area. This process of selecting sites ensures that exceptional teams run trials.
- Enhancing Diversity: AI tools also have the ability to identify and address the lack of diversity among trial populaces by examining demographic data so that they can pinpoint regions or sites that can involve underrepresented patient communities.
Predicting Trial Outcomes and Mitigating Risks
One of AI’s best features is that it can see ahead and predict problems.
- Forecasting Milestones: Key trial milestones, such as startup times, enrollment rates, and completion dates, can be precisely predicted by clinical trial predictive modeling. This allows for more realistic planning & resource allocation.
- Early Risk Detection: AI in clinical research can detect subtle patterns that may indicate future problems. AI-driven clinical research software can monitor real-time incoming information to detect future safety problems, supply chain gaps, or patient adherence problems, and act before they occur.
- Predicting Efficacy and Side Effects: Advanced models can even forecast a drug’s likelihood of success in later stages or possible side effects. This can assist in tough “go/no-go” decisions to be made much earlier in the trial, saving an untold amount of time and money.
Final Thoughts
The organizations that are willing to adopt these technologies stand to gain a competitive edge in a world of more complex and competitive clinical research operations. It is, however, not just the technology, but rather the organization that must be willing to adopt a data-driven approach, make the necessary investment in the technology, and associate with experienced clinical trial technology partners who understand the challenges and requirements of clinical research.
Are you prepared to use predictive analytics in clinical trials? To find out how our experience can enhance research results & expedite your clinical development programs, get in touch with us right now.
About Dash

Dash Technologies Inc.
We’re technology experts with a passion for bringing concepts to life. By leveraging a unique, consultative process and an agile development approach, we translate business challenges into technology solutions Get in touch.