The Future of Software as a Medical Device: What to Expect in 2025
Understanding Software as a Medical Device (SaMD)
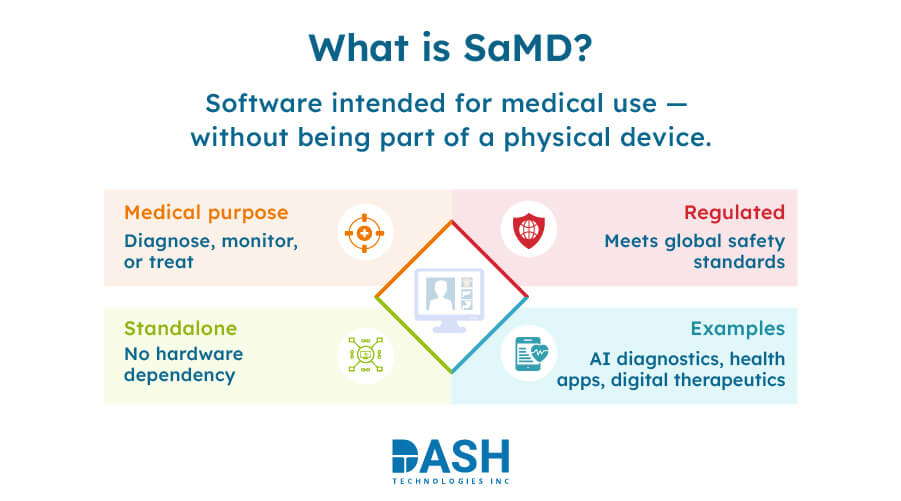
Software as a Medical Device is defined by regulatory bodies like the FDA and IMDRF as “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device.”
SaMD distinguishes itself from conventional medical device software, which is associated with proprietary hardware such as MRI machines using algorithms, data analysis, & artificial intelligence to deliver clinical results like disease diagnosis or chronic condition tracking through sensor readings and user input. SaMD is independent of proprietary hardware. The global SaMD market is projected to reach $5.0 billion by 2033, growing at a compound annual growth rate (CAGR) of 13.6% from $1.4 billion in 2023. This signifies that it is scalable and deployable on platforms like mobile apps, cloud infrastructures, and AI diagnostic platforms.
In contrast, Software in a Medical Device (SiMD) is integral to the functioning of a hardware medical device. SiMD cannot operate independently and remains essential for the device’s functionality. Some examples are software that operates the inflation process of blood pressure cuffs and software that manages insulin delivery through pumps.
Examples of SaMD include:
- Diagnostic imaging software analyzes MRI and CT scans for the purpose of detecting medical conditions, including strokes & tumors.
- Chronic disease management mobile applications enable patients to monitor their glucose levels.
- Artificial intelligence systems that help diagnose skin cancer through image analysis and predict potential heart attack incidents.
Regulatory Framework for Software as a Medical Device in 2025
The regulatory system for SaMD has transformed to support safety & efficacy while fostering innovation as digital technologies become more prevalent in healthcare. To ensure compliance and innovation, stakeholders engaged in medical device software development and integration software must understand the current regulatory framework.
United States: FDA’s Approach
In January 2025, the FDA shared a draft guide called Artificial Intelligence-Enabled Device Software Functions: Lifecycle Management and Marketing Submission Recommendations. The document presents a Total Product Lifecycle (TPLC) approach that details recommendations for developing, modifying, and monitoring AI-enabled SaMD aftermarket release. Key aspects include:
- Lifecycle Management: SaMD performance and safety depend on ongoing monitoring and regular updates through iterative improvements.
- Transparency and Bias Mitigation: This document provides guidance for developing strategies that will reduce biases in AI algorithms and promote fair results.
- Marketing Submissions: Recommending the essential documentation & information needed for FDA safety and effectiveness evaluation.
Build Compliant, Scalable SaMD Solutions with Dash
From regulatory-ready architecture to AI-powered functionality — Dash delivers secure, scalable, and compliant software solutions tailored for medical devices.
Partner with us to accelerate your product journey!International Medical Device Regulators Forum (IMDRF) Guidelines
The International Medical Device Regulators Forum (IMDRF) played a key role in standardizing worldwide regulations for SaMD. The final document, Characterization Considerations for Medical Device Software and Software-Specific Risk (IMDRF/SaMD WG/N81 FINAL:2025), was released by IMDRF in January 2025. This guidance provides a framework for:
- Risk Characterization: Potential hazards can be determined through analysis of intended applications and technological features.
- Device Characterization: The standardization of vocabulary and methodology for medical device software descriptions helps stakeholders communicate more effectively.
Integration of these considerations allows developers to effectively handle medical device software development challenges and meet global standards.
Adhering to International Standards
Central to medical device software development is compliance with international standards that ensure safety and efficacy:
- IEC 62304: This standard outlines the life cycle requirements for medical device software, emphasizing processes like software development planning, risk management, and maintenance.
- ISO 14971: ISO 14971 directs software developers for medical devices to apply risk management techniques that help them detect and minimize dangers throughout the product’s entire life cycle.
- IEC 82304-1: The standard establishes necessary safety and performance standards for health software products, which include medical device integration software that needs to fulfill quality benchmarks.
- Software Bill of Materials (SBOM): If you’re making software for medical devices, you need to provide a detailed SBOM. This document should list all the components, dependencies, and any third-party elements you used in your software. Being open about this info helps with managing vulnerabilities and keeps the software supply chain safer.
These standards combined act as a blueprint to create medical device software that is reliable & compliant.
Best Practices for Protecting Patient Data
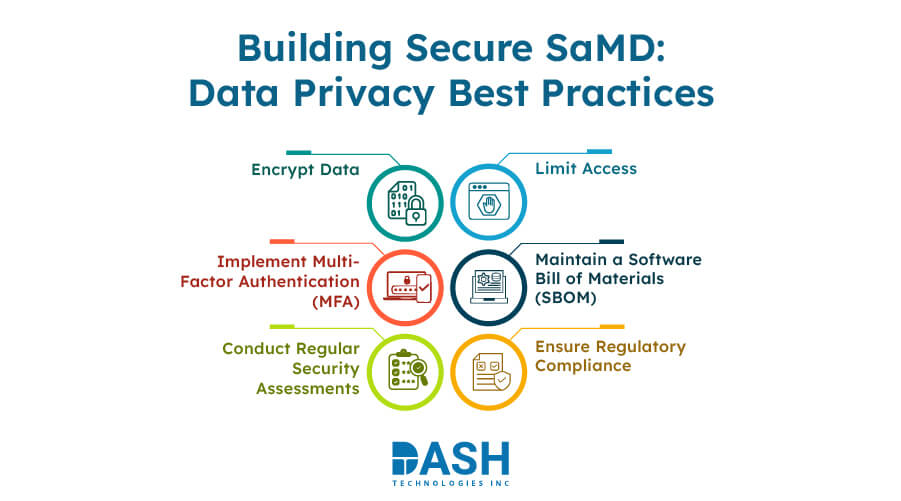
The protection of patient data in Software as a Medical Device (SaMD) is crucial in the year 2025. These fundamental practices provide the necessary steps to maintain data security and regulatory compliance.
- Encrypt Data: Protect data transfers and storage between software platforms through AES-256 encryption to ensure secure confidentiality.
- Implement Multi-Factor Authentication (MFA): Strengthen access security by implementing MFA requirements that minimize unauthorized entry into medical device integration software systems.
- Conduct Regular Security Assessments: Performing routine vulnerability scans and penetration tests allows for the identification and remediation of potential security threats in software.
- Limit Access: Implement role-based access controls within your software to eliminate unauthorized data access.
- Maintain a Software Bill of Materials (SBOM): An updated software inventory is essential for tracking all software components to manage vulnerabilities effectively.
- Ensure Regulatory Compliance: To fulfill international data protection standards, the medical device developers should comply with HIPAA and GDPR regulations as well as FDA guidelines.
The implementation of these best practices allows the developers & manufacturers to strengthen medical device software security while securing both regulatory compliance and trust of patients.
Future Outlook for SaMD
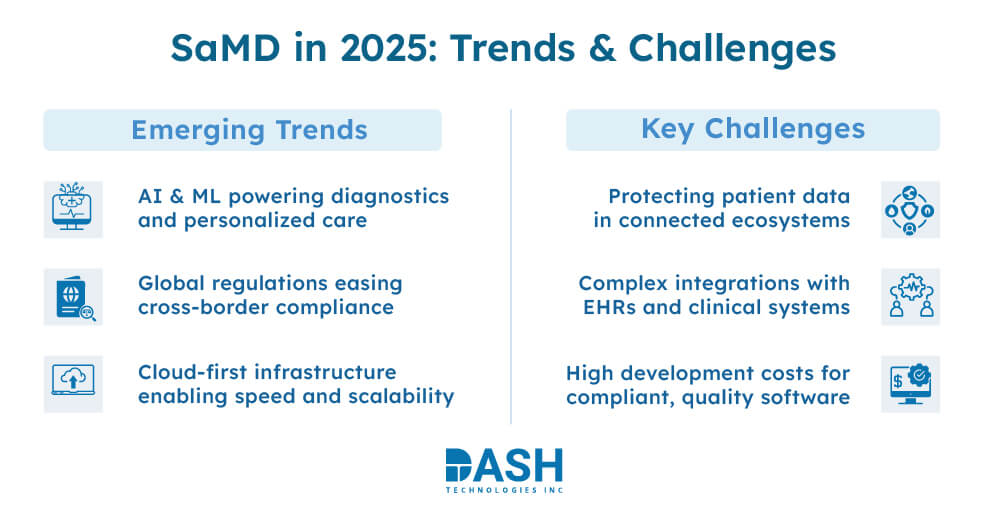
SaMD has a promising future, although it faces significant challenges. The following developments emerge in 2025 & continue:
Emerging Trends of SaMD in 2025
AI & Machine Learning Integration
Medical device software development will experience significant growth in AI/ML applications, which will power advanced diagnostics, personalized treatment plans, and predictive analysis software.
Global Regulatory Harmonization
The IMDRF works to establish worldwide unified standards that assist developers with easier cross-border compliance with medical device software development.
Cloud-First Infrastructure
Secure cloud platforms will become essential for SaMD solutions to achieve scalability and faster deployment, enhancing medical device integration software performance.
Challenges Ahead
Data Privacy & Security
All medical device software solutions recognize protecting patient data within a hyper-connected ecosystem as their highest priority.
System Interoperability
Medical device integration software requires seamless yet complex integration between SaMD and current EHRs and clinical systems.
High Development Costs
Startups face significant challenges when developing compliant, high-performing software due to the extensive need for R&D investment, regulatory filings, and quality assurance processes.
Final Thoughts
As healthcare transforms through digitization, Software as a Medical Device leads the innovation wave by changing the way medical data gets processed, diagnoses are delivered, and care is provided. From AI tools for diagnostics to software that helps different medical devices work together, the role of SaMD is only going to grow in the future. But, creating medical device software that meets regulations, is safe, & is scalable isn’t easy. It takes a lot of knowledge about both technology & the rules we need to follow.
Our expertise at Dashtech Inc. lies in converting challenging requirements into beneficial chances to create secure medical device software that surpasses FDA standards & IMDRF regulations alongside global benchmarks. Whether you’re refining AI-driven diagnostics, fortifying cybersecurity protocols, or optimizing interoperability, our end-to-end solutions ensure your SaMD initiatives thrive in 2025’s competitive market.
Have a project in mind? Let’s talk.
About Dash

Dash Technologies Inc.
We’re technology experts with a passion for bringing concepts to life. By leveraging a unique, consultative process and an agile development approach, we translate business challenges into technology solutions Get in touch.