February 18, 2026

Our Quality Assurance Team Leads You Through Regulatory Compliance Tasks
From assessment and document creation through supplier quality management and QMS implementation, DASH collaborates with you. Our quality assurance and regulatory compliance strategy guides you in assessing, planning, and executing phase-appropriate quality programs for starting, scaling, and sustaining operations.
Our team supports your familiarity with medical standards and the regulatory process. We analyze standards and regulations to establish design requirements based on identified specifications.
Risk management is crucial. Our quality assurance experts ensure ongoing risk analysis and management activities throughout the development process.
Get started nowOur Full Range of Quality Assurance & Regulatory Compliance Services
Rely on expert guidance to navigate the complex global regulatory landscape and stay ahead of compliance challenges. Our proven expertise, knowledge, and processes deliver flexible solutions to meet rigid compliance requirements, ensuring regulatory compliance without compromise.
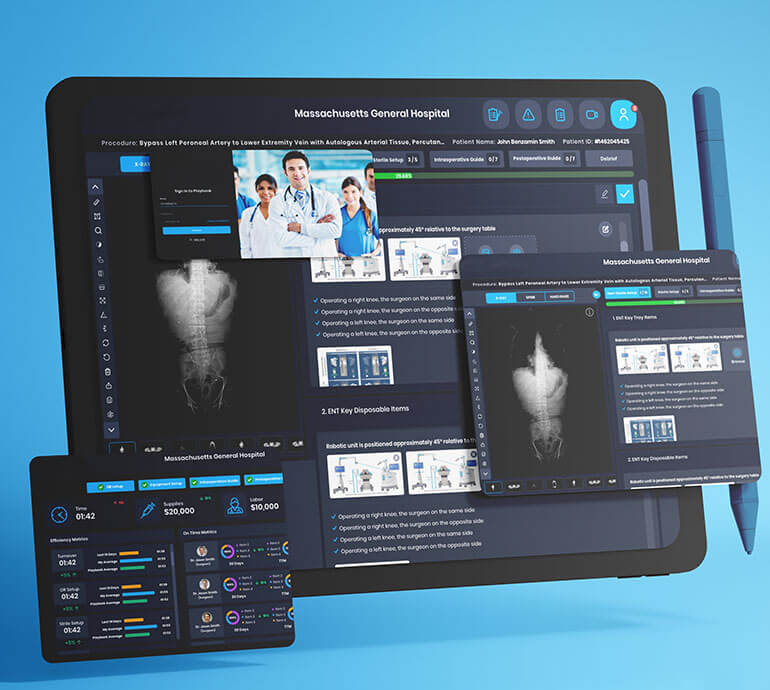
Device & Application Testing
- Multimedia & Audio/Video Testing
- Embedded System & Integration Testing
- Sensor Integration & Connectivity Testing
- Connected App & Edge Testing
- Product Performance & Functional Testing
- CI/CD & Device to Cloud Test Automation
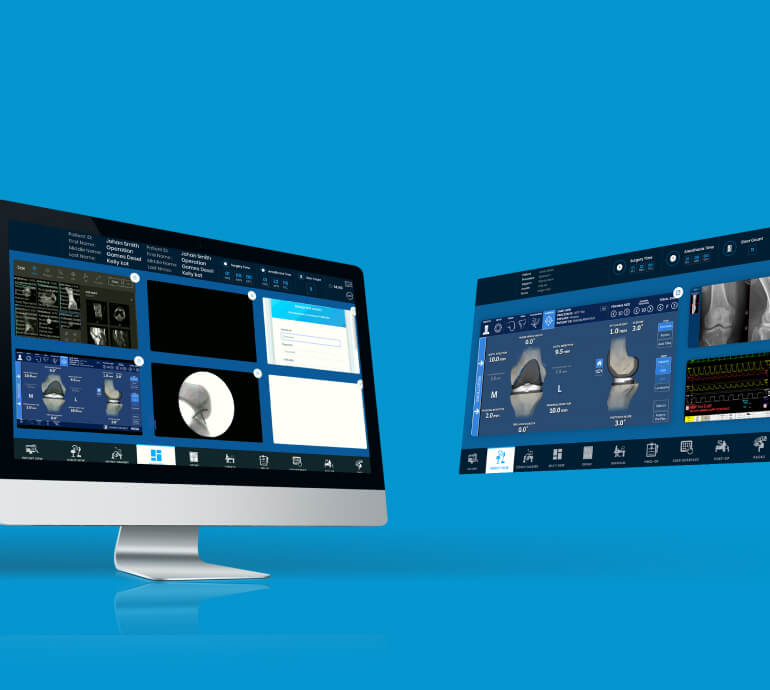
AI/ML Testing
- Dataset & Feature Validation
- Model Validation & Performance Benchmarking
- Model Parameters Traceability
- ML Library Integration Testing
- Embedded Neural Network Application Testing
QA Automation
- Python & Selenium scripts
- Automation tools & frameworks integration
- Load & Scalability testing with Jmeter
- CI/CD DevOps tools integration
- Test coverage tools integration
Certification, Compliance & Consulting
- IEC 62304 & ISO 13485
- FDA 21 CFR Part 11 & CE
- HIPAA, SOC 2, HL7, FHIR, HITRUST & GDPR
- Quality Management Systems Implementation
- Regulatory Compliance Audits
- Tools/Frameworks Assessment & Integration
- Risk Assessment & Mitigation
Let's Roll Together!
Achieve Quality Assurance & Regulatory Compliance
Our team of proficient QA, regulatory consultants, and business domain experts conducts thorough gap/impact assessments, steering change management seamlessly across the entire product lifecycle.
Find Out How Our Dedicated Regulatory Experts Can Help You?
Let’s talkOur Engagement Models
Tailored to suit your needs, our adaptable collaboration models offer short-term and long-term engagement options, enabling you to seamlessly scale resources as needed or control costs with sustained dedicated resources.
Project-Based
Flexible development services catering to a specific application or product, ensuring the achievement of milestones within predefined timelines, scopes, or budgets.
Dedicated & On-Demand Teams
Leverage our skilled engineers as your own by seamlessly collaborating to ensure flexibility and scalability throughout your development journey.
Fully Managed Solutions
Dash's cross-functional experts fully own the product development for you. Yielding accelerated delivery, high quality solutions and free up time for you to focus on your business.
Automate Business Processes
Our quality engineers prioritize automation for smoother product journeys, enhancing functionalities and operational efficiency to keep you ahead.

Our Tech Stack
Azure
AWS
.Net
C#
Python
Java
Apple
HTML 5
Case Studies
Explore our portfolio journey, where each project narrates a story of innovation, dedication, and transformative solutions.
Navigate Regulations with Expert Compliance Solutions! Let's Talk