Software Life Cycle Management for Medical Devices
Software life cycle management maintains medical device software security and functionality. It includes phases that help software meet evolving technology and regulatory requirements. Proper management keeps software secure, compliant, and updated while reducing risks. Following these stages helps organizations address issues early for better outcomes.
What is Software Life Cycle Management?
Managing the development and maintenance stages of medical device software is important. It ensures the software meets requirements and functions properly during development and use. The life cycle involves planning, development, testing, maintenance, and decommissioning. It manages updates and issue resolution. In medical devices, software failure is critical. It can impact patient safety. It ensures compliance with FDA and ISO standards while keeping the software secure as technology advances. Managing the process of software helps firm lower risks and increase device reliability. This results in better health assistance.
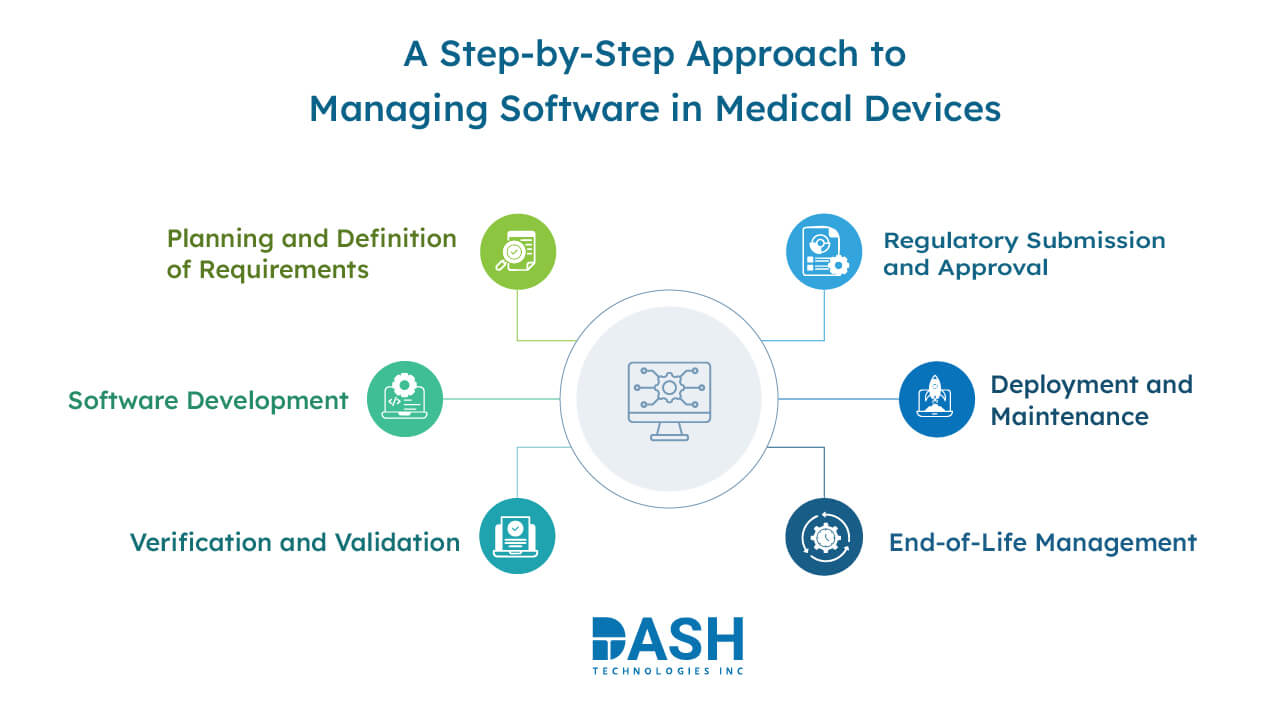
Key Stages in the Management of Software for Medical Devices
-
Planning and Definition of Requirements
Identifying system specifications, regulatory requirements, and software needs forms the initial stage. Developers, regulatory teams, and healthcare professionals work together to define well-documented objectives. Documentation at this level establishes the compliance and risk management basis.
-
Software Development
Developers design the software following pre-defined specifications. Coding practices adhere to best practices in the industry, incorporating secure coding mechanisms and software testing. Version management and documentation make it traceable and maintainable during development.
-
Verification and Validation
Testing guarantees that the software is as expected and works perfectly. Verification validates whether development happens by requirements, whereas validation ensures the final product meets the needs of the users. Unit testing, integration testing, and system-level validation are involved in this stage.
-
Regulatory Submission and Approval
Medical device software must comply with FDA and industry standards. Companies submit regulatory materials explaining software design, test outcomes, and risk evaluations. Regulatory approvals enable the deployment of software in healthcare settings.
-
Deployment and Maintenance
Once approved, the software is rolled out for implementation. Maintenance entails bug fixing, security patching, and optimization. Ongoing monitoring guarantees that the software remains dependable, secure, and up to date with regulatory changes.
-
End-of-Life Management
When the software life cycle comes to an end, organizations have to plan decommissioning. Secure data transfer, risk avoidance, and correct documentation ensure an easy transition. Retirement plans avoid disruptions and ensure regulatory compliance.
Transform Your Software Development Journey
Unlock the full potential of your business with tailored software solutions, scalable apps, and expert engineering services.
Explore More!Quality Management in Medical Device Software Development
Quality management is vital in developing a medical device software solution. It ensures safety, meets compliance standards and maintains performance. Medical software must function without failure because any error can impact patient care. A clear quality management process helps companies spot risks early. It helps them solve problems while reducing costs and meeting regulations.
A robust quality management strategy involves ongoing testing, version control, and change documentation. Risk analysis is essential to avoid failures and gain regulatory approval. Updates and security patches ensure the software remains secure and functional. Focusing on quality at every stage helps organizations boost software reliability. It also makes getting regulatory approval easier and enhances patient support.
IEC 62304: Importance in Medical Device
IEC 62304 is the global standard employed to create software for medical devices. This provides the method of regulation and safety in the stages of medical device software development. It implies creating, maintaining, and managing the software efficiently. It standardizes the maintenance and development of medical device software. This renders the software safe as well as follows performance requirements. They need to comply with IEC 62304 to be approved by the regulators. This includes FDA clearance.
IEC 62304 compliance enhances product reliability for companies. It also simplifies regulatory approvals and enhances patient well-being. It also facilitates risk management throughout the software lifespan. This is founded on hazard identification, analysis, and control. IEC 62304 ensures software updates and modifications maintain security and compliance.
Meeting IEC 62304 helps companies create reliable products. It reduces approval times and improves patient security. The standard also facilitates interoperability with other health systems. It reduces long-term development costs through the elimination of faults in the initial stages. Compliance builds trust with regulators, doctors, and users. It results in efficient and safer medical technology.
Top 3 Challenges in Managing Software Throughout Its Lifecycle
-
Maintenance of Software Updates:
Software needs to adapt to new technologies, user needs, and industry changes. It is difficult to deal with updates without disrupting operations. Unmanaged updates cause compatibility issues and system failures. Therefore, a scheduled update plan will provide smooth changes that satisfy user demands.
-
Bug Fixing and Tracking:
Bugs can emerge at any stage, making early detection and resolution essential for software quality. If bugs are not tracked and tested, they can accumulate. This can lead to significant system crashes, causing damage to user satisfaction and operational effectiveness.
-
Security Management:
Software security management is becoming increasingly difficult with every new threat. Regular security checks keep the system free of vulnerabilities. To avail effective prevention against data breaches, cyberattacks, and similar security risks, requires efficient methods. Vulnerability scans should be performed regularly so that software integrity and user trust can be guaranteed.
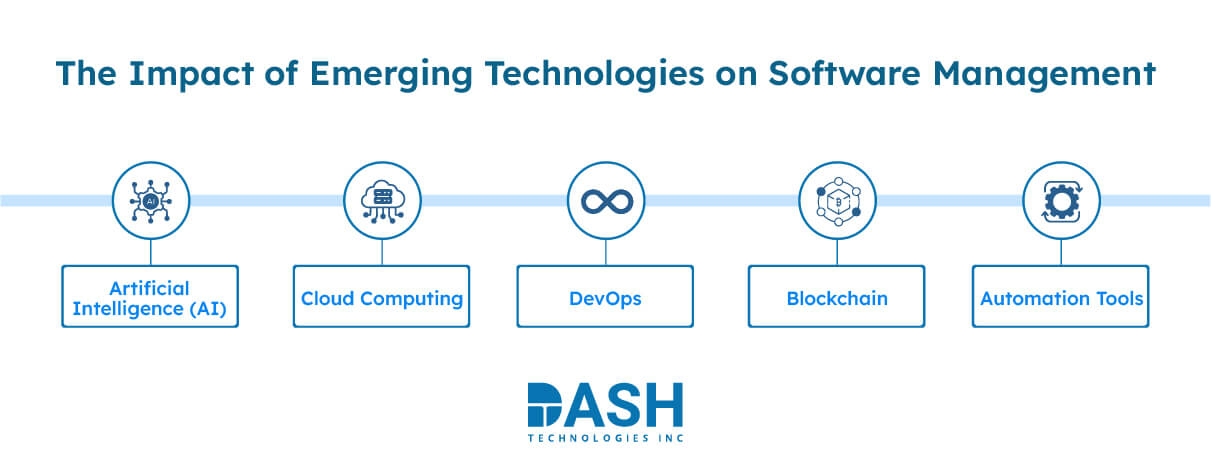
Transforming Software Management: The Role of Emerging Technologies
-
Artificial Intelligence (AI):
AI enables bug detection and code analysis to be automated in medical device software development, enabling teams to catch issues early. It saves time and accelerates problem-solving, allowing teams to focus on more complex issues.
-
Cloud Computing:
Cloud computing offers flexibility in that software can be executed on remote, elastic infrastructure. It enables remote access, seamless updates, and team collaboration.
-
DevOps:
DevOps aligns development and operations in a way that allows easy pushing of updates and quick resolution of bugs. It reduces errors and speeds up the deployment of software through automated integration and delivery.
-
Blockchain:
Blockchain is capable of protecting software by tracking change in an open, distributed fashion. Blockchain enforces data consistency, ensuring unauthorized changes become more challenging to miss, as this serves to maintain confidence within software systems.
-
Automation Tools:
Automation tools eliminate manual tasks like testing and deployment, making them more effective. They make the software consistent and reliable, leaving teams to work on refining features and fixing higher-priority bugs.
Why Choose DASH Technologies for Your Software Engineering Needs?
DASH Technologies offers customized software solutions that enable companies to automate processes, innovate, and grow successfully. With specialization in cloud development, mobile applications, and full-stack engineering, we walk you through the complete software development process to facilitate smooth integration, peak performance, and long-term success.
Key Areas Where DASH Technologies Delivers Value:
- Custom Software Development: We design solutions that meet your business needs at every stage of the software development journey.
- Cloud & Mobile App Development: Our expertise helps you build scalable apps that are ready for the future.
- Full-Stack Engineering: We specialize in both front-end and back-end development to create end-to-end solutions.
- Risk Management: We deliver secure and reliable software to ensure your business remains operational.
DASH Technologies delivers secure, scalable, and innovative software solutions. Connect with us to navigate the software life cycle and unlock your business’s potential.
About Dash

Dash Technologies Inc.
We’re technology experts with a passion for bringing concepts to life. By leveraging a unique, consultative process and an agile development approach, we translate business challenges into technology solutions Get in touch.